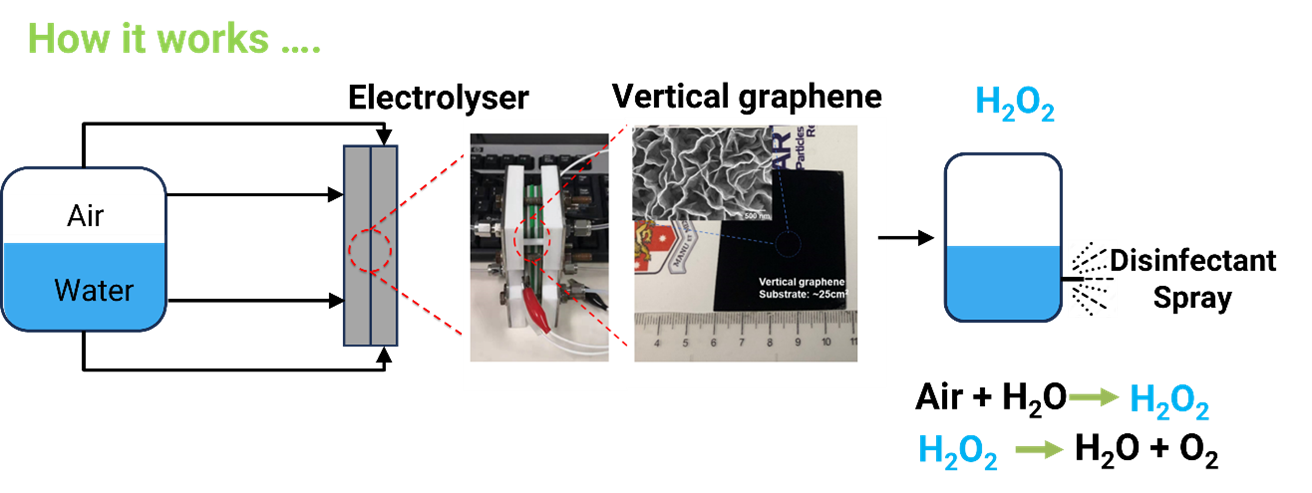
Chemical engineers apply principles of green chemistry and engineering to design processes that minimise waste, reduce energy consumption and prioritise the use of renewable resources. The chemical industry is one of the main contributors to CO2 emissions from industrial processes. Hydrogen peroxide (H2O2) is one of the most versatile and cleanest chemicals in the world, with applications span various industries including paper and pulp bleaching, chemical synthesis, food processing, agriculture, wastewater treatment, healthcare, mining, semiconductor and aerospace industries. Currently over 95% of H2O2 is produced through the traditional anthraquinone process, which requires large-scale infrastructures, substantial energy inputs and generates wastewater and CO2 emissions. The industrial process typically produces high concentration of H2O2 (>27.5%), although typically most end users require concentrations of less than 3%. The electrochemical generation of H2O2 enables decentralised, on-demand production using only air, water and electricity as input, without any hazardous byproducts.
The Peroxiguard novel device is capable for onsite production of hydrogen peroxide solutions with a wide range of concentrations (~ 0.05 wt% to 2 wt%) via the electrochemical oxygen reduction reactions. Coupled with electricity generated from renewable energy resources, this technology is a solution for on-site and on-demand green hydrogen peroxide production.
Enterprise Academic Fellow Ding Zhang’s groundbreaking research has the potential to disrupt the hydrogen peroxide industry. Supported by TRaCE, Ding will develop a safer, more sustainable method for producing hydrogen peroxide H2O2 through chemical synthesis.
Connect with Dr Ding Zhang to know more about green H2O2 production.
