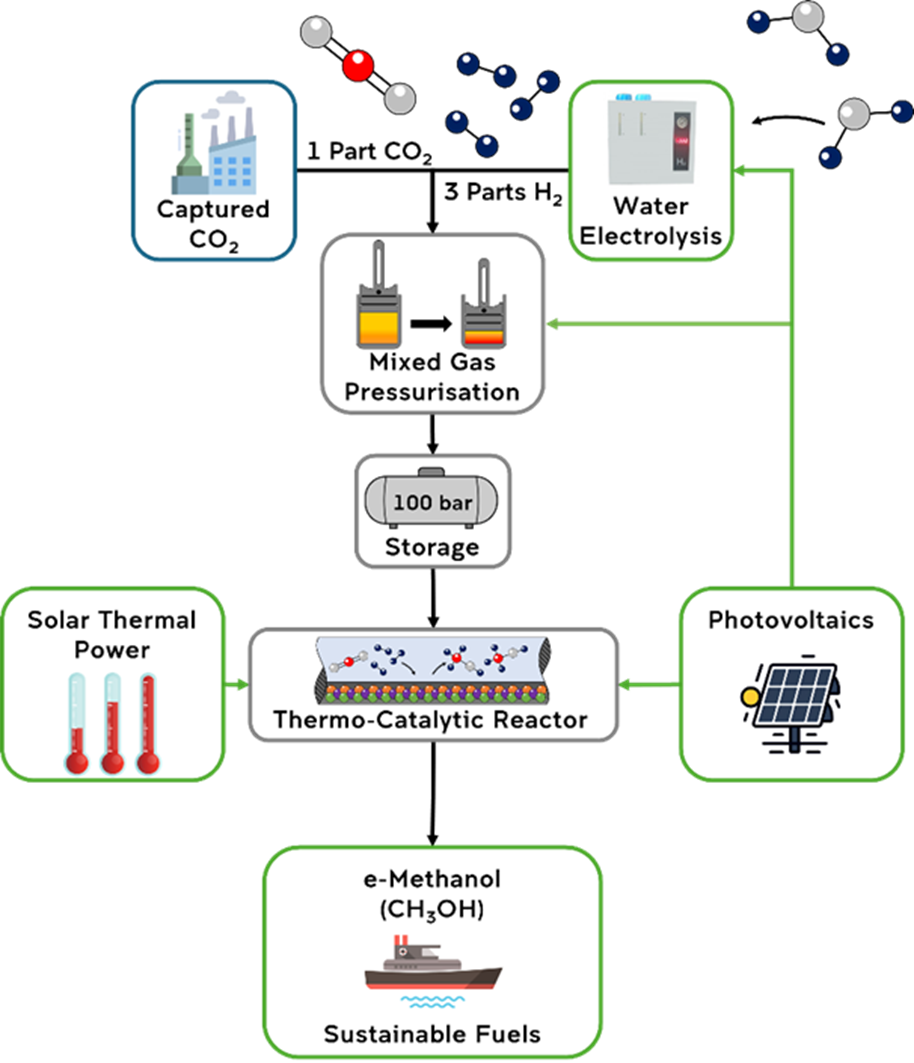
In transitioning away from traditional fossil fuels, the generation of e-Methanol is a promising Power-to-X pathway. e-methanol is a biodegradable, clean burning alternative fuel source with significant decarbonisation potential. When used as a maritime fuel, a noticeable reduction of generated SOx, NOx, and particulate matter emissions relative to traditional diesel combustion is observed. Aviation industries, through the incorporation of e-Methanol as a precursor for synthetic aviation fuel, also presents a significant opportunity for clean energy initiatives. A crucial benefit of e-methanol is the ability for the safe integration into existing infrastructure, fast-tracking decarbonisation effects. The Particles and Catalysis Research Group is focusing on demonstrating how solar energy can be harnessed for the selective generation of e-methanol in SHINE 3.0.
In a previous study, PartCat has successfully reported the synergistic excitation of a copper / zinc oxide / alumina (CZA) catalyst with ultraviolet and visible light to facilitate the generation of methanol.
CO2 + 3H2 → CH3OH + H2O
Building on these works and the new understanding of the reaction pathway, PartCat has developed a pilot-scale system in SHINE 3.0 to demonstrate the synthesis of e-Methanol. Carbon dioxide (CO2) is stoichiometrically mixed with hydrogen gas (H2) before the mixture is pressurised up to 100 bar. The CO2 used represents a captured source from industry, with the H2 generated onsite through the solar driven electrolysis of water. Solar thermal energy is concentrated to heat the thermo-catalytic reactor up to 300 °C. In conjunction with the effects of elevated pressures, e-Methanol is successful generated and captured in a liquid form with a conversion efficiency of 60%.
A significant focus of this project is to effectively utilise solar energy in multiple forms. Photovoltaics allows for the operation of SHINE 3.0 independent from external grid input and harnessing of thermal energy through a solar concentrator to heat the reaction chamber significantly reduces the overall energy requirements for e-methanol generation. Further research will target advancements in the system regarding improved photoactivation of the CZA catalyst as well as development of new catalysts and refined reaction conditions to increase selectivity and the relative yield of e-methanol, supporting the transition of e-fuels to industry.